OUR NANOFLUIDIC TECHNOLOGY REVOLUTIONISES POINT-OF-CARE IN VITRO DIAGNOSTIC SOLUTIONS
Abionic’s nanotechnology is transforming today’s blood-testing standards by bringing laboratory quality to near-patient testing and simultaneously analysing up to 14 parameters from a single drop of blood, to provide actionable results in just 5 minutes.
By driving molecules into a nanochannel and limiting the travel distance to a few hundred nanometres, molecular interactions are accelerated rapidly. Thus, biomarker levels of e.g. antibodies and proteins can be efficiently quantified within an extremely short assay time, with high precision and accuracy on a closed, small and easy-to-operate platform.

Unparalleled technology offers patients the fastest point-of-care diagnostic testing solutions based on traditional immunoassays.


The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the quantitative measurement of pancreatic stone protein (PSP) in blood. The test is intended to be used in conjunction with other clinical assessments and laboratory findings to aid in the early detection of nosocomial sepsis in adults.

The IVD CAPSULE D-Dimer is a single use, rapid in vitro diagnostic test for the quantitative measurement of D-Dimer in venous whole blood. The IVD CAPSULE D-Dimer is intended to be used in the aid in the diagnosis of venous thromboembolism (VTE) including deep vein thrombosis (DVT), pulmonary embolism (PE) and disseminated intravascular coagulation (DIC), in patients suspected of DVT, PE or DIC. D-Dimer testing is often ordered in the primary care and emergency room (ER) with symptoms of a serious condition (e.g., chest pain and difficulty in breathing).
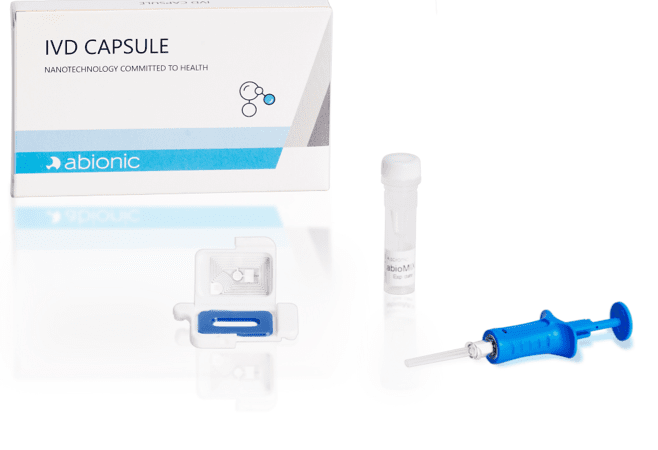

The IVD CAPSULE COVID-19 is a rapid, single-use in vitro diagnostic test that measures the presence of a specific SARS-CoV-2 viral antigen in nasopharyngeal or saliva sample. Abionic’s COVID-19 test allows for ultra-fast screening of COVID-19 patients at the point-of-care with lab quality results.
- 100%
- 95.1% for Ct values <25
- 93% for Ct values <30
- 92.8%
Saliva

The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the determination of Pancreatic Stone Protein (PSP) in blood for the calculation of the cSOFA score at the point of care. The cSOFA score is used to assess the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in adults.

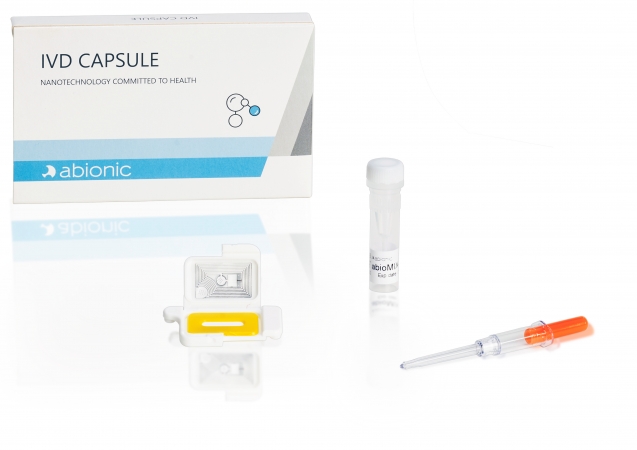
The IVD CAPSULE Ferritin is a rapid, single-use in vitro diagnostic test for the quantitative measurement of ferritin in capillary and venous whole blood, to aid blood diagnostic testing for iron deficiency in adults.
The IVD CAPSULE Aeroallergens is the first rapid, single-use in vitro diagnostic test for the quantitative determination of circulating immunoglobulin E (IgE) specific to the allergen components Phl p 1 + Phl p 5 (combined), Der p 1 + Der p 2 (combined), Alt a 1, Fel d 1 and Can f 1 in uncoagulated human capillary blood collected from the patient’s fingertip.
Order now
Are you interested in having the abioSCOPE right at the bedside of your patients? We’d love to hear from you. Get in touch with us just below to request an offer!
